Sodium cocoylisethionate (SCI) is an anionic surfactant, which is biodegradable and safe for the natural environment. Its most frequent forms are sticks, granules and powder.
Classification of surfactants:
Surfactants are compounds whose structure contains a hydrophilic part and a hydrophobic part. The hydrophobic part has affinity to oil phase, whereas the hydrophilic part shows affinity to water phase.
Surfactants can be classified in terms of their function in the finished product, e.g. as an emulsifier, cleansing or foaming agent, solubilizer, or dispersant agent.
Another classification groups surfactants by their origin – from renewable substances (plant oils, proteins, simple sugars) or from non-renewable raw materials (hard coal, crude oil).
However, the most frequent division is into ion- (anionic, cationic, amphoteric) and non-ion surfactants.
SCI is an anionic surfactant, i.e. an amphophilic compound. These compounds dissociate and are biodegradable. Their most frequent application is in cosmetic products.
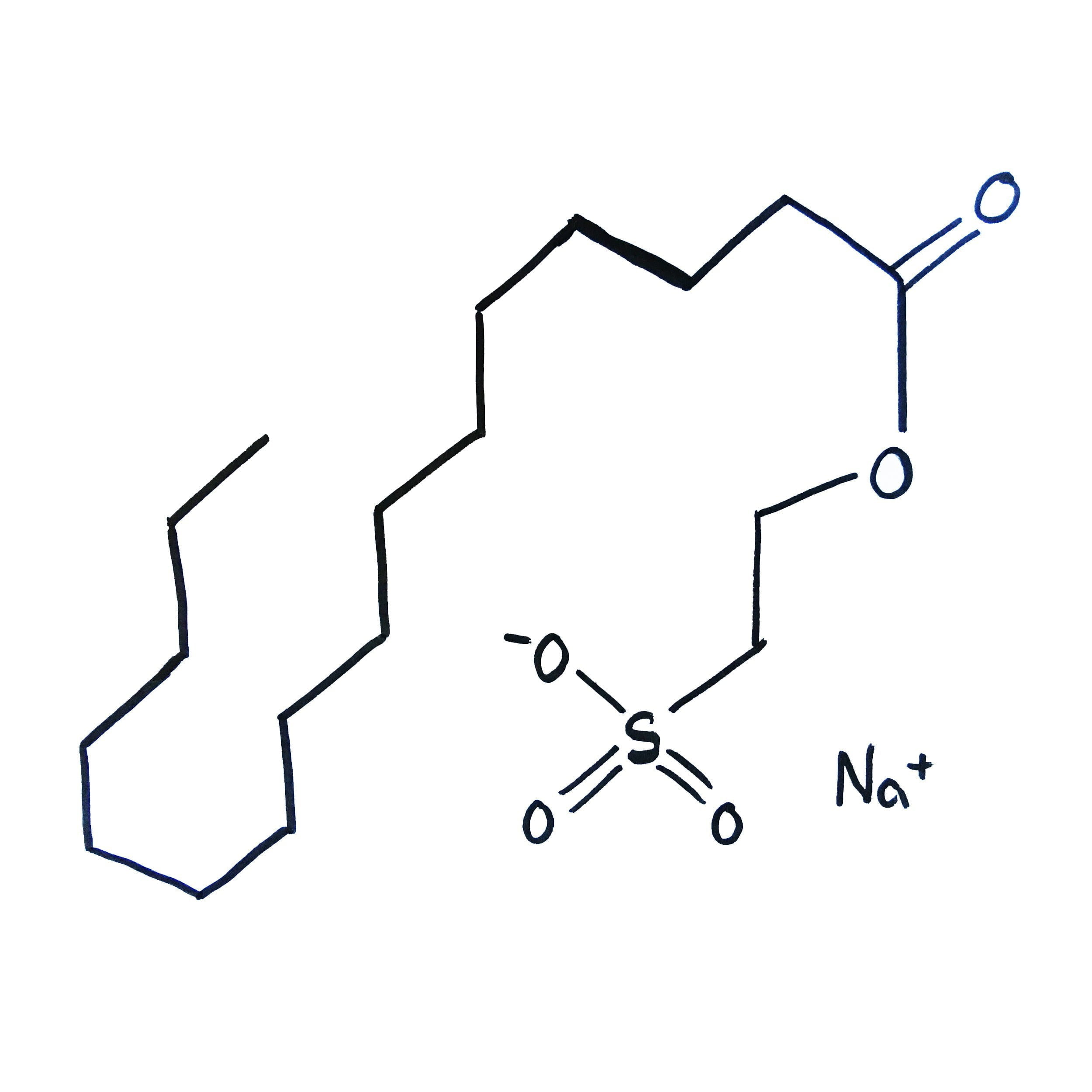
SCI (Sodium Cocoyl Isethionate)
We are a wholesale distributor of SCI (Sodium Cocoyl Isethionate) – an anionic surfactant used in safe and natural cosmetic products.
This substance is completely safe for human health and the environment.
SCI properties:
SCI – sodium cocoylisethionate, is a substance naturally derived from coconut oil. It contains fatty acids and the sulfonic (isethionic acid). To maintain its properties, SCI requires proper storage conditions – in a cool place, away from light and heat. SCI is safe for external applications. SCI has been a subject of many research studies and has not been found to cause any significant adverse effects. It is considered safe for use in cosmetic formulations.
However, to make sure no allergic reaction occurs, a quick test may be conducted: Place a small amount of the product containing SCI on your hand and wait for a moment, looking for any significant changes. If no irritation develops, the product can be used as instructed.
Applications of SCI in cosmetic products
Sodium cocoylisethionate is a mild surfactant for hair and skin. Upon contact with water, it creates a pleasant, thick foam. In cleansing products, it cleans and smoothens the skin without causing any irritation or dryness. SCI has a moisturizing, softening and smoothing effect on skin and hair. It also has emulsifying properties, giving cosmetic products a creamy texture and increasing their viscosity. In hair care products, it may decrease hair tangling and make it easier to comb. SCI may be used in mild cleansing products for persons with sensitive or allergy-prone skin.
Sodium cocoylisethionate dissolves dirt well, binding impurities and leaving the skin clean and moisturized. It retains its effectiveness in both soft and hard water, which makes it useful for a wider range of cosmetic applications. Thanks to its chemical structure, SCI has many beneficial properties that make it delicate even for baby skin. Therefore it is a frequent ingredient of bathing products for children. SCI is biodegradable and causes no harm to the environment, so products with this ingredient are a recommended choice for any natural and ecological routine.
Cosmetic uses of sodium cocoylisethionate:
SCI is used in make-up removal and personal hygiene products, as well as plant-based cosmetics. Applications of SCI in cosmetics include:
- Shampoo bars,
- Cleansing bars,
- Peeling bars,
- Bath balls,
- Shampoos,
- Foam baths,
- Shaving gels and creams,
- Bathing products for children,
- Make-up removal milk.
Summary:
The contents of SCI in cosmetic products should be in the range of 3-20%. As sodium cocoylisethionate is a mild surfactant, if looking for a product that is delicate for the skin and does not cause any irritation, it is best to choose a formulation in which SCI is a leading ingredient. Importantly,SCI is biodegradable and does not cause harm to the environment.
Bibliography:
- https://www.newdirectionsaromatics.com/blog/products/all-about-sodium-cocoylisethionate.html
- p00559-p00568-with-cover-page-v2.pdf (d1wqtxts1xzle7.cloudfront.net)
- Otrzymywanie i właściwości użytkowe nowych surfaktantów z ugrupowaniem cukrowym. Katarzyna Michocka – Praca doktorska, Poznań 2012


